The energy sector is witnessing a fundamental shift away from fossil fuels and towards low-carbon alternatives. Countries around the world are aiming for zero emissions by 2050 and green hydrogen has been identified as a major enabler in securing a sustainable future. Its ability to convert, store and transport energy derived from other sustainable sources, such as wind and solar power, while producing zero emissions makes green hydrogen a valuable solution in the global energy transition and we are starting to see the green shoots of global hydrogen markets starting to take hold.
HOW CAN GREEN HYDROGEN CHANGE THE WAY WE USE ENERGY?
The key hydrogen technology in the production of green hydrogen is Water Electrolysis.
Using energy from renewable sources, Water Electrolysers generate ‘green hydrogen’, by splitting water into its component molecules, Hydrogen (H2) and Oxygen (O2), resulting in zero greenhouse gas emissions. By using this process of extracting hydrogen when there is excess energy in the grid – particularly on windy or sunny days, green hydrogen can be produced from renewable sources and stored cheaply for later use. Fuel cells or turbines can then convert the stored hydrogen back into electricity when it is needed.
HOW DO WATER ELECTROLYSERS WORK?
An electrolyser is a system that facilitates electrolysis - the breakdown of water into oxygen and hydrogen.
Electrolysers vary in form and function and, depending on the application, can be scaled up or down to produce energy outputs, but the process is essentially the same. In its most basic form, an electrolyser consists of an anode and a cathode separated by an electrolyte. In polymer electrolyte membrane (PEM) electrolysers, the electrolyte is a solid specialty plastic material, known as the ‘Proton Exchange Membrane’.
- Water reacts at the anode forming oxygen and positively charged hydrogen ions (protons)
- The electrons flow through an external circuit and the hydrogen ions selectively move through the PEM to the cathode.
- At the cathode, hydrogen ions combine with electrons from the external circuit to form hydrogen gas. Anode Reaction: 2H2O → O2 + 4H+ + 4e- Cathode Reaction: 4H+ + 4e- → 2H2
* TFP Hydrogen materials are used in the Porous Transport Layer (PTL) and we produce a range of anode and cathode catalysts.

HOW CAN GREEN HYDROGEN BE USED AS A MAINSTREAM ENERGY SOURCE?
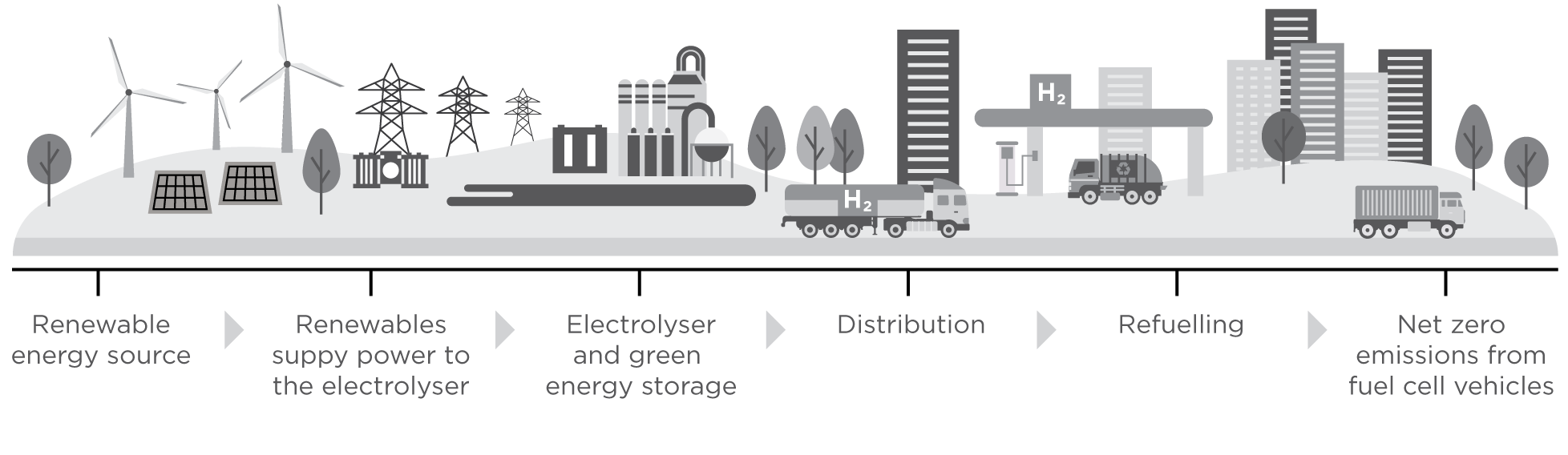
With it’s Net Zero credentials, high purity and ability to be used as an energy source in a wide range of applications there is no doubt that green hydrogen is an attractive alternative energy source to advance the energy transition. However, to make the mainstream use of hydrogen a reality, a dedicated infrastructure needs to be in place and the technology used to generate it must demonstrate both performance and afforability.
WHAT WORK IS TFP HYDROGEN DOING WITH WATER ELECTROLYSERS?
At TFP Hydrogen Products we are committed to facilitating the advancement of electrochemical, low carbon technologies that can be used to generate green hydrogen from renewable energy sources.
We offer solutions for the three most common types of water electrolysers where our specialist coatings and high performance catalysts are proven to significantly increase the efficiency, durability and lifetime of the systems:
- The nature of PEM electrolysers makes their components highly susceptible to oxidation, corrosion and hydriding, reducing stack performance and impacts their functionality and lifespan. We have extensive experience within this field and have specifically developed specialist coatings for Porous Transport Layers (PTL) which improve the efficiency and durability of PEM water electrolysers – effectively reducing the cost of green hydrogen production and for catalysts.
- Alkaline water electrolysers are the most established form of water electrolyser technology. Our team has developed highly active cathode catalyst materials, combined with proven durability. Our catalysts are relevant for technologies with Ni electrodes, further reducing energy consumption in operation.
- Anion Exchange membrane (AEM) electrolysis is one of the latest types of water electrolysis and is rapidly becoming of increased research interest globally. It provides a means of producing high purity hydrogen from sustainable sources which has potential cost and material advantages. AEM electrolysis offers potential benefits in reduced capital costs. This can be achieved through the use of low cost non-platinum group catalysts and non-critical raw materials. However, there is a lot of development still required in order to realise these cost and scalability benefits and make AEM electrolysis an essential player in the goal to achieve net-zero CO2 emissions by 2050.
- We are currently collaborating with several partners as part of the Anione Project, a research and innovation project that will develop high-performance, cost-effective and durable anion exchange membrane (AEM) water electrolysis technology and make the technology accessible. Find out more about The Anione Project here: Concept - ANIONE
